Technical Report on counterfeit Heliocare
Technical Report with findings on counterfeit Heliocare sunscreens detected on the market.
It has came to our attention that there are presence of counterfeit Heliocare in the market. To address this issue, counterfeit Heliocare sunscreens were dispatched to Cantabria Labs’ facilities, where a thorough comparative evaluation was conducted. This evaluation was prompted by disparities observed in the packaging, which raised concerns about the authenticity of these products.
A technical report has been provided to Neoasia, sole authorised distributor of Heliocare in five markets, including Singapore. This technical report shows the results of the comparison between both products (original product produced in the facilities of Cantabria Labs vs counterfeit product) from the points of view of Quality Control and Packaging.
A comparative study of both products has been carried out. Firstly, a visual assessment has been made, where the Quality Control and Production departments analyzed both the characteristics of the box and packaging, comparing them with those standards defined by Cantabria Labs. After completing this step, Quality Control proceeded to evaluate the contents, including an analysis of the level of photoprotection, which is the primary characteristic of genuine Heliocare sunscreen. It’s important to note that using suspected counterfeit or fake products may pose health risks if they do not provide the essential function of a sunscreen product, which is photoprotection.
Comparison between the packaging
- Front side
|
Image 1. Front view of both products (box and tube) |
Comparing both products, the following differences are observed:
- At the box level, the color tone of the letters and the SPF 50+ logo differ from the one approved by Cantabria Labs for this product.
- At the tube level, it is observed that the letter format and the location of the product batch do not coincide with the standard format approved by Cantabria Labs (Product 2).
2. Bottom
The lower part shows data that help to track the traceability of the products manufactured by Cantabria Labs. Image 2 shows that the batch number used in Product 1 does not follow the batching system established in Cantabria Labs for its cosmetic products.
|
Image 2. Bottom of boxes |
3. Back side
When checking the back side, and looking at Image 3, it can be seen that Product 1 does not have in none of the sides the closing tamper of the box approved by Cantabria Labs, unlike Product 2 (yellow circle). In addition, the coloured part where the way of obtaining additional information of the product does not coincide with the one selected by Cantabria Labs.
About the tube of the product, several significant differences have been detected. It is clearly observed that the size and font used is different; as well as the location of the batch number on the tube. In Cantabria Labs the batch of the product “HELIOCARE 360 WATER GEL” is placed in the upper area of the front face (Product 2), while Product 1 has it located in the upper part of the back side of the tube (see lower part of Image 3).
|
Image 3 Back side of both products (box and tube) |
Formula-level study
Quality Control has conducted a study of both product formulas, evaluating their in vitro SPF (the main function in a sunscreen product) and organoleptic properties (color, texture, odor).
Organoleptic properties
Product 1 does not meet the organoleptic characteristics set by Cantabria Labs.
Firstly, when opening the products, it has been detected that Product 1 does not have the corresponding smell associated with the “HELIOCARE® 360 WATER GEL” distributed by Cantabria Labs.
With regard to colour and texture, a comparative sample of both products is presented in Image 4. The first significant difference is the colour and viscosity, where Product 1 has a yellowy colour that does not comply with the specifications established by Cantabria Labs for its product, as well as a higher viscosity, as it is not a gel.
In addition, differences have been observed when spreading the products, with Product 1 showing more surface coverage (Image 4).
|
Image 4. Differences in colour and extensibility between the two products |
Sun Protection Factor (SPF)
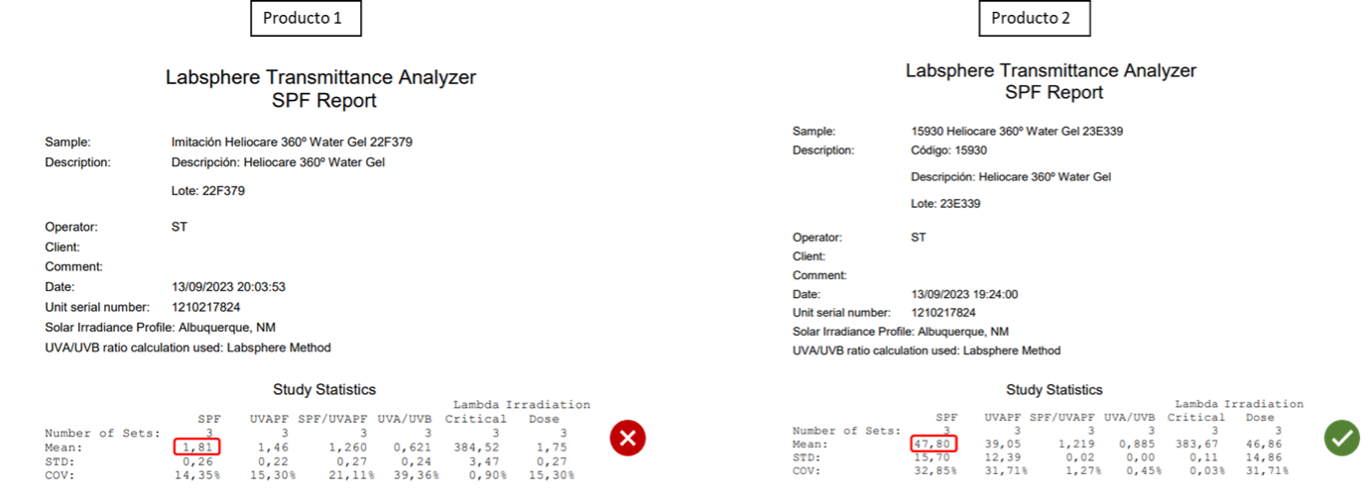
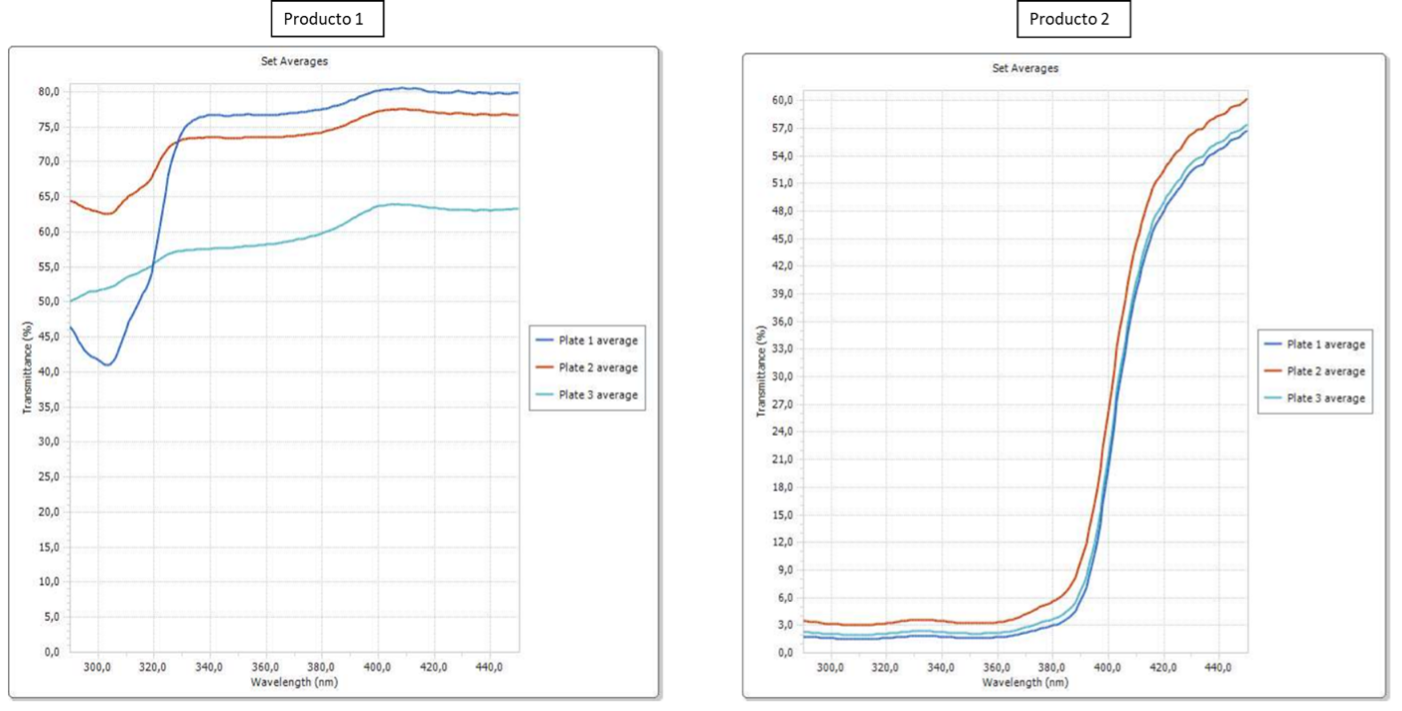
Images 5 and 6 show the data and graphs obtained in the in vitro SPF test carried out on both products.
|
Image 5 In vitro SPF results. |
|
Imagen 6 In vitro SPF chart |
Analyzing the results obtained, Product 1 does not have the photoprotective effect in contrast to the product “HELIOCARE 360 WATER GEL” produced in Cantabria Labs, as it does not absorb in the UV range (280 – 320 nm). As photoprotection is the main function of our product “HELIOCARE 360 WATER GEL”, non-compliance with this requirement is a very high risk for consumer safety.
Upon analyzing the results, it is evident that Product 1 lacks the photoprotective effect observed in ‘HELIOCARE 360 WATER GEL’ produced by Cantabria Labs, as it does not absorb in the UV range (280 – 320 nm). Given that photoprotection is the primary function of our product, ‘HELIOCARE 360 WATER GEL’, its non-compliance with this requirement poses a significant risk to consumer safety.
In conclusion, following the comparative study at both the packaging and formula levels, we can confirm that Product 1 is not the original product manufactured in Spain and marketed by Cantabria Labs. It does not adhere to the labeling and fails to fulfill its intended primary function, thereby jeopardizing consumer safety. Consequently, we strongly urge consumers to exercise vigilance while shopping and seek peace of mind by choosing trusted sources.
Purchasing Heliocare from unauthorised sources could lead to health risk and complications. Buy only from authorised clinics, websites, and stores. If you’re unsure of the authenticity of your Heliocare products, please contact us and we’ll be happy to advise.